Lead acid batteries often die due to an accumulation of lead sulphate crystals on the plates inside the battery, fortunately, you can recondition your battery at home using inexpensive ingredients.
A battery is effectively a small chemical plant which stores energy in its plates. They are chemically charged with an electrolyte which is a mixture of distilled water and sulphuric acid. When the battery is discharged, the lead active material on the positive plates reacts with the sulphuric acid and produces lead sulphate. When the battery is charged, this process is reversed and the lead sulphate crystals react to form sulphuric acid again. The battery fails when there is an excess build up of lead sulphate crystals which then do not allow sulphuric acid to make contact with sections of the plate. These crystals harden and eventually cause a chemical imbalance in the electrolyte.
In most cases, hardened crystals can be removed using a solution of magnesium sulphate. This method doesn’t restore a battery back to original condition but it will restore it to around 70-80% of its original capacity and can be repeated, allowing you to get a few more years of use out of your battery without having to replace it.
What You Will Need To Recondition Your Battery
- The Damaged Battery
- 400ml (12oz) Distilled Water – Buy Here
- 200g (7oz) Epsom Salts (magnesium sulphate) – Buy Here
- A Syringe or Dropper – Buy Here
- A Battery Charger – Buy Here
How To Recondition Your Battery
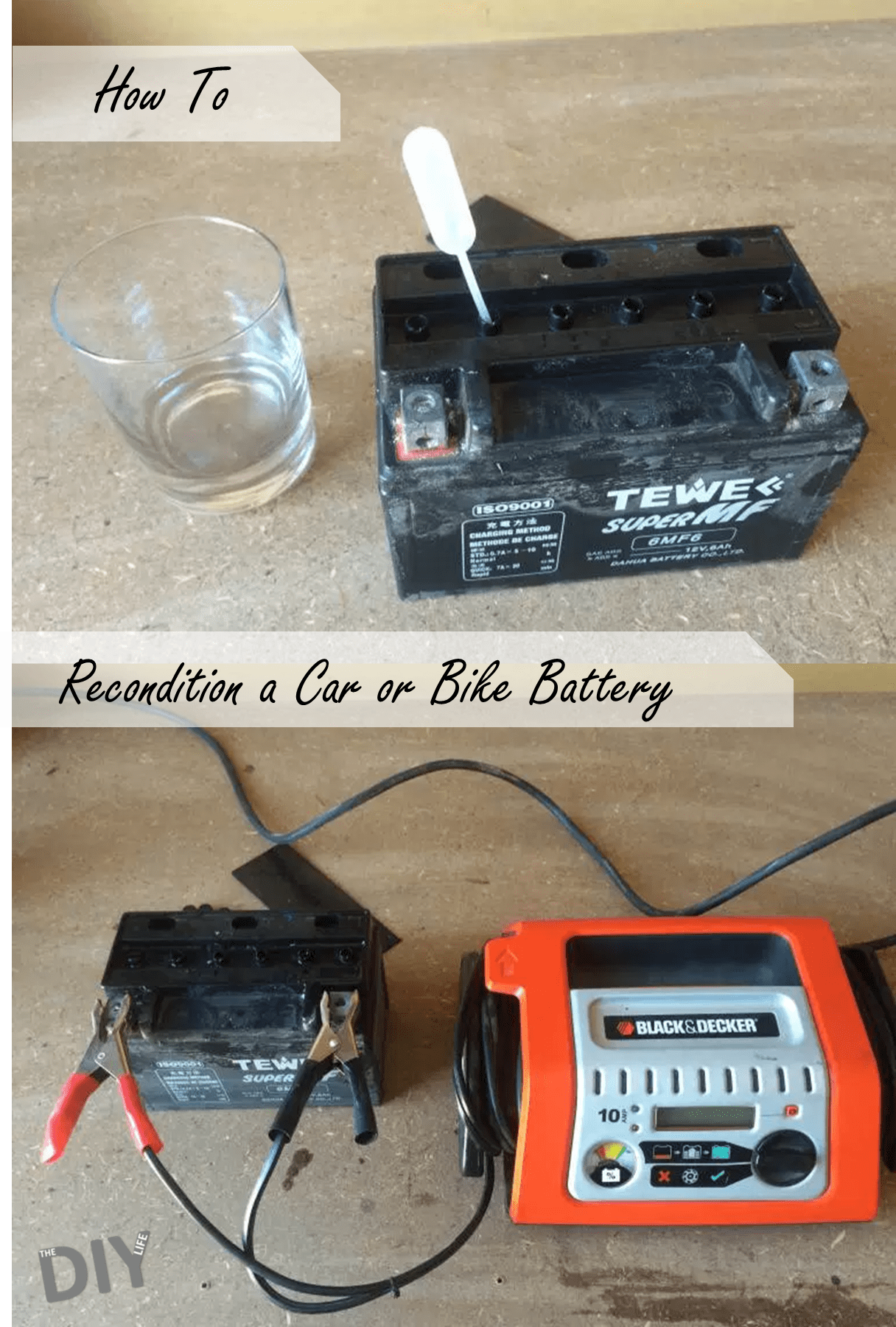
Take the battery out of the vehicle, motorbike or scooter and put it onto a solid work bench.
Some battery’s cells are clearly visible on top of the battery and are sealed with screw in caps. Others, like mine, are protected by a “sealing” strip. You may need to cut the edges of this strip to get it loose but it is almost always removable. Look for the edge of this strip and try to pry it up using a flat screw driver, if it is glued into place, try to cut around the edges of the strip using a sharp craft knife.
Once this has been removed, you will also need to take the caps off each of the individual cells in order to get to the battery acid. Some batteries have small rubber caps like these, others (typically on larger batteries) have screw in plugs which can be removed with a large screwdriver, they’re not usually very tight.
Using a syringe or dropper, carefully drain each cell one by one until they are all around 50-60% full, if some cells are already lower than this then exchange some acid from the fuller cells. You don’t want to take too much out as you will then struggle to charge the battery again. The liquid you are removing is a strong acid so put it into a glass container and be careful not to mess any of it on your hands or clothing.
Make sure that you dispose of the removed battery acid in a safe and responsible manner. The removed battery acid is extremely corrosive and contains heavy metals, mainly lead.
Now you need to make a saturated solution of Epsom salts (magnesium sulphate) and distilled water. Do this by boiling water and continuously stirring in more salts until no more will dissolve in the water. Then fill each cell with the Epsom salt solution to the full level line using the syringe or dropper.
When charging the battery while it is being reconditioned, some gas will be released, so it is advisable to leave the caps open. Connect a battery charger to the terminals and let it complete the charging cycle. If the battery is heavily drained or damaged, it may have to be charged overnight with a trickle charger at a very low amperage. If you do not have a battery charger then replace the battery cell caps and covers and reinstall the battery in the vehicle. Jump start it and then take it for a full hour or two drive to allow the battery to charge using the alternator.
The reconditioned battery should now last another 6 months to a year and can usually be restored using this method about three to five times until it is no longer effective.
Edit: As some users in the comments section have suggested, the best solution would be to let the reconditioning process run for a few days to properly “clean” the plates and then drain the Epsom salt solution from the battery and replace it with the recommended 35/65 acid solution. Make sure that your battery is discharged before removing the Epsom salt solution and replacing it with the acid solution.
Have you tried to recondition a battery using this method or a similar method? Let us know in the comments section below.
Share this guide with your friends:









About 15-16 months ago I added distilled water to my cars completely dead battery. I happened to have a charger that loved to overcharge, causing the batteries electrolyte to boil. I continued to overcharge and top of the fluid (in a vented area, of course). This was a test to see if it would clean hardened lead sulfate from the internal plates to extend its life. The battery lasted till this winter before drastically losing its capacity.
So I’d like to safely create my own solution of battery acid mixture – just like humanity did when lead acid batteries were serviceable in the early days. I don’t see that topic covered too often.
i think you are right to over-charge the battery, this is called voltage equalisation by some people, and the extra voltage seems to be able to reduce the sulfate deposit.
I red some good methods for reconditining batteries, we can do it at home and save a lot of money. No need to throw out old batteris and i beleive these methods will helps to start a new business also. I belive this is a good guide (step-by-step guide).
Can ordinary table salt be used to recondition a dead car battery .
Hi Otto,
No you can’t use ordinary table salt. Table salt is chemically very different to Epsom salts and the process involves a chemical reaction to clean the plates within your battery which won’t work with table salt.
How do you discharge the battery ?> the n you can poor out the distal water/ espson water solution?
thank you
No. Epsom salt is not the same as table salt.
MgSO4 (epsom salt or Magnesium Sulphate) vs CaCl (table salt)
H2SO4 (sulfuric acid)
PbSO4 (Lead Sulphate)
The idea is to use MgSO4 a highly soluble salt to pull the PbSO4 crystal off the plate.
Table salt is sodium chloride (NaCl) not CaCl
very accurate !
Anonymous,
If I was to empty the battery of all liquid then fill it with a distilled/baking soda mixture and let it sit for 4 hours, would the baking soda mixture pull the Lead Sulfate Crystals off of the plates? (Or does that process specifically require Epsom salts?)
I would then empty the battery of the mixture and flush it with water, then with distilled water thoroughly before adding a store bought 65/35 mixture of Sulfuric Acid. Then slow charge.
No, this process is a chemical reaction which requires Epsom salts and electricity (the battery charger) to work.
The baking soda works better, and its faster working.
What is the ratio of gypsum salt and water to be used in
The baking Soda is Alkaline, which nutralises the Acid. It will not dissolve the Sulfate. I have done a lot of reading on chemistry, and any chemical that can dissolve Sulfate will also dissolve the Lead in the battery.
So, the use of baking soda in this case, is only to reduce any burning of skin, wen one empty out the battery acid.
Good post.
I’m working on my military truck m929a2 batteries. I’ve bought and sold 14 of the 5 tons and each has (had) 4 of the big 6tl, dead from sitting. This truck is the first that would start under its own power, but lately no. Removing them there was very low corrosion, cells full, ranging from 10.5v to 11.5. I brought 2 inside to warm up and recondition. My charger is small, has 2 and 10 amp settings.
The 2 that I’m working on are holding an open circuit at 12.35 and 12.45 so far. I understand these Exide batteries are full if they hold at 12.9.
I’ll reread the procedure before I do them. Thanks.
Hey Tjoes. I know this is an old post but it caught my attention because of your mention of the military vehicles. I just got two m932A2 Tractors on a trade, so I need info on them. Specifically where can I buy parts and manuals? Got lots of air brake issues, scary when you know nothing about them. One has got 21,800 miles, the other 2,500 miles – relatively new. Any advice would be appreciated.
Cheers, Wayne
No it cannot you would ruin the battery
I initially posted back in January. Since then, I’ve done a lot of research and have done a bit to recondition several of the batteries I have that hold a poor charge. I attempted the Magnesium Sulfate method (Epsom Salt dissolved in distilled water). This is only a temporary solution to allow electrical current to pass through the hardened lead sulfate insulator. It also helps break up the lead sulfate once it accepts the charge and is properly recharged. However, it seems as if the magnesium sulfate shortens the life of the battery afterword.
I began to (using proper PPE) siphon the discharged sulfuric acid from a couple cells and noticed that the plates were breaking in to chunks. So the plates themselves are very fragile apparently. That battery was definitely no good at that point. The local automotive stores here sell battery fluid acid (30-38% sulfuric acid) in a 6 quart box sealed inside of a HDPE (plastic) bag. Generally, certain HDPE’s are storage for sulfuric acid. My next effort is to clean out a battery that has plates in good condition. Safely of course.
Hi Jack,
Thank you for the great feedback. It would certainly be interesting to do some testing on a range of new and reconditioned batteries to determine the effect on the life of the battery after performing this “reconditioning”. I’ve done this process on two or three batteries now and they’ve all lasted around a year after the re-conditioning although they have all been the smaller motorbike batteries as shown in the images here. Perhaps a larger vehicle battery degrades faster.
Please let us know how your cleaning out exercise goes, I’d love to include a follow up with some images on what you’ve found!
The say lead acid battery is 99 % recyclable?
I’m not sure about the exact percentage but they are one of the most recyclable battery types.
The sulphuric acid is typically neutralised and turned into sodium sulphate, which is used to make fertiliser and detergent. The lead and plastic components are then recycled into new batteries or other industrial products.
What are the quantities used in making the magnesium sulphate solution ( Epsom salt and water)
We have a vw polo 6R. The battery died and went to test it. They said we must buy a new one. After seeing this post we’ve tried it on the old battery. And it is working like a charm. Thank you for your advise and saved us some money
Hi Pieter,
Thanks for the great feedback! I’ve found that I can get at least a year or two of additional life out of my motorbike batteries using this process, it’s definitely saved me a lot of money over the years!
Hi I’m going to give it a go tomorrow but after I’ve completed the epson salt thing I’m going to put fresh acid solution back in the battery
The battery will go to full charge but just doesn’t have any guts
I hope I’m not disappointed
Cheers stu
Hi Stu,
Going to full charge but not producing any current makes a pretty good candidate for this reconditioning process. You can replace the battery acid eventually but rather leave the epsom salt mixture in the battery for a few weeks to assist with breaking down the lead sulfate crystals. As soon as you remove the epsom salts, you’re stopping the crystal removal process as well. Good luck.
Agreed ,I’ve done it Several time on 40-50 batteries , Most took 3-4 weeks if charge/discharge after which I drained and rinsed the battery and replaced the battery acid and recharged again ..Some were working 10 years after production in a vehicle…
No need to add fresh acid solution to the battery. The sulfuric acid in the battery breaks into sulfur which reacts with the lead to make lead sulfide. Epsom salt is magnesium sulfide and has the same sulfur that the acid does to react with the lead. It is just as effective.
thats a very interesting concept,i live off grid so i have a relatively large battery bank plus a few older battaries from an earlier system that sat for a long time (about 3 years). i was looking for a good proceedure to reclaim these older battaries. they are deep cycle (golfcart 6 volt units) but are traditional lead acid. youre serious that epson salt can be used instead of acid in these rebuilt units?
Yes.
i did the same think with the epsom salts with no luck im going to drain it out and put new acid and recharge and see how it goes
How do I safely dispose of the old acid I’ve removed from the battery?
This is an excellent post with good comments. Thanks all!
Hi Denise, thanks for the great feedback. Fill a bucket with water and then slowly add the acid to the water while stirring. After that add a teaspoon of baking soda at a time again while stirring until you stop getting a bubbling reaction. This should neutralize the acid and it can then be poured out in a bare patch in the garden or down the drain.
This reply is correct, but it’s important to state that you should never add water to acid (only the other way around). The reaction is very different and extremely dangerous.
then please explain adding water to a battery, thats water going into acid.
The “add acid to water, not water to acid” is not about the chemical reaction (which can’t change based on direction) but about splashing during the mixing. The liquid being poured displaces the liquid in the receptacle which can splash up/out of the container and injur someone or damage surrounding surfaces.
While this is still true when adding water to a battery full of acid, there is no choice of direction in this procedure and the small opening of the battery cell limits the risk.
It sure is about the chemical reaction. Water has a higher heat capacity to hold heat generated by the reaction with acid. More Initial water Volume with Smaller acid Volume added doesn’t overload the the heat capacity of water. Done backwards, acid has less capacity to Dissipate heat from The reaction with added water causing extremely unstable heat from the reaction and possible explosion if too much water is added to an acid. The old chem lab motto for us was “do as you outta, add acid to watah (water)!
The chemical fact is as follows: Sulfuric acid has a great affinity to water specially when highly concentrated and it will jump out of its container towards the water when you pour the water on it causing splashes and burns, however in the case of a car battery this concentration is not high enough to cause that, having said that you should always pour acid onto water where possible.
He’s just being a safety nut cuz that’s what we learned in chemistry class and he’s theoretically right. However in The real world with a battery you could never accomplish this You have to add water to acid. Some people just like quoting safety there’s a couple guys like this at work. They have to quote everything out of a book like it’s a Bible to them.
Doesnt this nutrilized mixture still contain dissolved lead and is thus toxic?
Yes, you are contaminating the soil.
You will not neutralize the lead.
I would ask your local government recycle center as how to dispose of the solution .
Hello Everyone. Thanks for all your advice. Does anyone think this procedure can work with a sealed 200Ah Gel battery?
no.
Add enough baking soda which will neutralize the acid. A lot of gas will be produced so do this in an open area.
Its like taking baking soda when you have stomach acid reflux. You start burping!
Can you use the acid you took out of the battery and use it as a drain for as net to unclog drains?aren’t there commercial drain cleaners that are made with sulfuric acid?
Hi Bill,
There are quite a few commercial drain cleaners which are sulfuric acid based, these would however be used by individuals who are qualified to work with it. If you do use it, use it with extreme caution. It is very corrosive and there are a number of safety risks which need to be properly managed.
when all steps are finished can we need to refill lead acid ? please reply me. Thank
It’s not essential but will result in better performance afterward.
I haven’t sharpened up on my battery knowledge lately but I’ll explain what I can recall. Once the mixture of h20 and sulfuric acid are injected in to the battery and an electrical charge is introduced across the terminals, the hydrogen and sulfuric acid chemically react with the batteries lead plates.
The sulfate ions become negatively charged while the hydrogen ions become positively charged. What happens when the battery is losing its charge? Through chemical reaction, the sulfuric acid begins to react with the lead plates and forms in to lead sulfate (the white powdery substance you may see on a battery terminal).
So if my memory serves me right, a well discharged battery would have lost its initial acidic strength. I have removed the “electrolyte” from a battery but I did not stick my fingers in there to test the level of acidity.
So yes, you probably could clean out a drain with battery “electrolyte” if the sulfuric acid is present. Is it going to work as well as drain cleaner? No. The sulfuric acid content in a battery is 30-38%. Some drain “uncloggers” are advertised as 95% sulfuric acid.
Lead sulfate is also hazardous. That’s something you definitely wouldn’t want to dump down your drain and introduce to the water system and environment.
Hi The DIY Life i would like to know that after i desulfate the battery should i leave the Epsom salt electrolyte inside or i mus remove it and put fresh sulphuric acid? And if so how long must i leave Epsom salt in the battery after reconditioning?
Thanks Regards
Hi Tinashe,
Once you’ve run through the slow/trickle charge process and your battery is fully charged again. Remove the liquid from the battery and replace it with fresh sulphuric acid for the best results. You only need to leave it in for a full trickle charge cycle.
Ok thank you i have done my battery today so tomorrow i must remove the liquid? And after taking that liquid out must i put it on charge again when i put new sulphuric acid
Yes, if your battery charger has finished charging the battery tomorrow then you can remove the liquid. You can put it on charge again after swapping out the sulphuric acid, it will probably be almost fully charged already anyway.
Ok cheers i will do that thanks again
Last year I performed this process on a 7 year old battery that would not start my truck. The plates were smothered. The process worked and if fact the battery seemed stronger than it was when new. I worked in the industry of battery chargers for led acid batteries for 4 years. I’m very familiar with charge, float and gas stages of battery maintenance. My company developed a very special and unique stage know as pulse charging. It’s like hitting the plates with a hammer to crack and weaken the dendrite crystals formed during the sulfation phase. Even with my background I never knew you could do this! I doubted it would work. I think its just something the battery industry want’s to keep secret.
I’m started the process on the same battery after the truck sat for 9 months without charging. I will let you know how it turns out. Theoretically as long as the plates and the plate doping are in decent shape there should be no limit to how many time the process can be performed.
I did two of my batteries now i didn’t come right and i don’t know where i lost it can you please send the whole process of how its done and measurements of Epsom salt with water
Keep up good work guys
Hello, very interesting post. I know that, when charging a battery, a good indicator that the charge is working is the generation of small bubbles (of hydrogen if I’m not wrong). Last night I was charging my car’s battery and I noticed that one cells is not making any bubbles, is this because of hardened crystals or is it another cause? Thanks in advance.
Hi Xavier,
Yes that sounds correct although there shouldn’t be excessive bubbling, just a few tiny bubbles. It could be due to crystallization or through corrosion of the cell connections or some other problem with the cell. It only really tells you that the cell isn’t charging properly, you can’t really determine why without taking the battery apart.
right , if its bubbling more than a glass of cola would its cooking. i know more about battaries than most as i have lived on them for almost 8 years (solar). but there are still things i can learn thank you for the info.
Xavier should throw that battery out as a defective cell cannot be resolved. It is probably shorting out due to the piling up of debris from the plates on the cell floor, thereby touching the lower plate extremity.
I have heard of washing out the debris and refilling to spec and recharging.
Can we use use sulpheric acid to recondition the old battery. I want to recondition sealed lead acid 6v 12v batteries. What happen if I use sulpheric acid instead of distill water and epsom salt.
Hi aakash Epsom salts must be used to create the necessary chemical enviroment for the decrystalization of the plates to be a success.
I drained my motorbike battery that did no longer held a charge. Then I filed it with 110% seawater and charged it for about an hour. It tested 12V. Instaled it in my bike and it stated after about a minute cranking. the next day it stated as if the battery was fully chaged.
how long will this work with the seawater electrolite..?
Hi Alternativejp,
I have never heard of anyone intentionally putting seawater into a battery and I’m not sure why yours worked, chemically it doesn’t really make sense. Passing any electrical current through salt water is not the best idea as it produces chlorine gas.
Sorry, I just have to state the obvious. A few points, firstly, see water and battery cells in contact, major corrosion. If your battery had legs, it would run away. That’s a very dangerous procedure. Secondly, no need to drain and replace the epsom salt solution. It’s a very good conductor and more environmentally friendly than other toxic additives. I have been reconditioning and restoring batteries for some time and I make good money in doing so. I have restored batteries from 0 volts. No charger will accept 0 volt battery however you can use a full battery connected to the 0 volt battery with jumper cables. Leave it for a hour and then measure the voltage on the 0volt battery. It sure has more than enough volts to be charged . Thank you for a great read and some handy tips which i completely agree with. Kind regards, Johan
It works. . . I measured voltage of a 12 volt battery at 5 volts before unloading the old solution until the top of the plates. I then added the epsom salts solution and measured the voltage. It increased to 12 volts even without charging. Wonderful . . . Thanks for sharing .
.
There is a man on the internet who swears by first filling the battery with soda and distilled water, charging, emptying and repeating until all the black runs clean. He said the charging was important to clean the plates. Then after rinsing with distilled water, he did the Epson salt with distilled. He said he has done completely dead batteries. He put it back in his riding lawnmower and it started right up. He seemed to think the soda charge was the most important cleaning step.Cayman
Please alert me to any new posts in this thread. Thanx
I am going to try this today…I will let you know how it goes! My husband thinks I’m crazy!
How do you tell when the battery is 50-60% full? i have 4 – 12v batteries. Thanks
A lead acid battery is around 50-60% full when the unloaded voltage is around 12.20 volts.
Question and confirmation regarding “discharge then recharge” after “reconditioning”.
I have a portable photography battery pack that uses 12v lead acid batteries which have now died. The batteries are in enclosures which slide into the photo power pack for easy battery change out. I removed the battery from the enclosure and added distilled water to the cells but they will not hold the 12v recharge. Reading your post it is my understanding that I must “recondition” the cells, however, I am concerned about the gasses and discharge/recharge while in the enclosure AFTER “reconditioning”. I just want to confirm that I must now:
A) Remove the liquid in the battery and neutralize with baking soda for disposal.
B) Add the distilled water and Epson Salt mixture for reconditioning
C) Charge the battery full
D) Remove the reconditioned liquid in the battery and neutralize with baking soda for disposal
E) Add ONLY distilled water and recharge
Again, my concern is the use of the battery and possible gases AFTER the “reconditioning” since the caps would now be back on the battery and battery is placed back into the enclosure.
Other posts seem to recondition the battery and leave the same liquid in the battery after the process. Since the battery is constantly discharging and recharging one would think that gasses would again be produced and cause possible damage. Any comment or direction is appreciated.
Your basic understanding is correct. Depending on the severity of the deposits in the cells, you may want to run them through a discharge and recharge cycle two or three times with the epsom salt solution before emptying them out and replacing the distilled water.
With regards to the gases. Try keep them open and in a well ventilated place when doing the reconditioning. When you’ve put the distilled water solution back into the battery they’ll be fine again. For your application I wouldn’t suggest leaving the epsom salt solution in the batteries as it does generate a lot more gas when charging. Lead acid batteries, although sealed, do have small ventilation holes to release gases generated when charging or discharging.
Great article and comments. There are some discrepancies between the many modified instructions in the comments. Sometimes it’s recommended that after some time reconditioning the battery with the epsom salt solution to then remove that solution and then restore the battery “fluid” with an acid solution. Other times the recommendation is to (after reconditioning) replace the epsom salt solution with just distilled water. I’m having a hard time believing that if replacing either the acid or epsom salt solution (after reconditioning) with 100% water that the battery fluid will ever return to it’s normal acidity (35% sulfuric acid / 65% water). Should not the replacement fluid after reconditioning be the 35/65 mixture or some other ratio? Is the correct ratio dependent on other factors like the condition of the plates, etc? All comments/suggestions are appreciated. Thanks.
I think the best method is to recondition the battery wit the epsom salt solution and then remove the solution after some time and replace it with the recommended (35/65) acid solution. Simply replacing the solution with distilled water will lead to very poor battery performance, as you’ve stated. In theory you can also simply leave the epsom salt solution in the battery, although performance will be slightly lower than if you replace the solution.
After reading all the posts, the last one by the OP seems to make the most sense… and is the one I will try
what i don’t understand is how the chemical reaction of converting lead sulfate. to sulfuric acid is supposed to take effect once you remove the sulfuric acid and replace it with (epsom salt) Magnesium sulfate. to my understanding the epsom salt just helps clean the stuck lead sulfate off of the lead plates and converting some of it back into sulfuric acid.
it sounds to me that we will have a low concentration of sulfuric acid left in the battery.
would it be better to clean the inside of the battery with epsom salt like you show, but then to drain it, and replace the sulfuric acid with new acid?
Yes you are correct John. As per some of the other comments here, the best solution would be o replace the sulphuric acid afterwards.
Have tried to recondition my N70 with Epson and when I tested it was
showing 12v but it can not start my car what could be the problem and how can I fix it?
Hi Luguza, did you retain some of the battery acid during the process? Did you also complete a full charging cycle with a trickle charger?
I read somewhere that short bursts of charge at 18Volts high amperage is a method used by some experts to dissolve lead sulphates… in my experience all these methods either epsom and 18v had never worked, perhaps my epsom concentration is to low, (seems to shorten the life of my batteries) and the 18V leave me only dead batteries, bikes and cars batts all died… perhaps I am doing everything wrong and all these battery methods must be extremely precise to work.
for charles i do equalizing on batteries its shouldnt be equalized no more than 16 volts otherwises ull short out the battery 5 mins on 10 mins rest repeat 5-6 times
Slight variation. I took all the acid out then washed out the battery with tap water thoroughly. Put hot mixture of Epsom and distilled water in, 1kg salts and 2L distilled water for around 20m shaking the battery a little and put onto charge for a few minutes. Then decided to poor out the salty water and put the old acid back in minus the settled out solids. Charged for 15Hrs then put into car. Cracked over pretty well.
Thanks for sharing your variation with us. Glad you’ve got good results from it!
Hi
What is the Ratio of the salt and the water?
Thanks
Correct Way Of Reconditioning battery:
1. Pour out all the OEM electrolyte and save it in a plastic container
2. Dissolve 250g per Liter Epsom salt in 60°C distilled water
3. Trickle charge The battery till full. – The Mg2+ ions will reduce (disolve) the Crystalline PbSO4 that is stuck on the plates (Mostly the -ve plates).
Pure lead (Pb) will be deposited on -ve plate & Lead Peroxide (Pb02) on the +ve plates.
Electrolyte specific gravity will also rise due for formation of H2SO4 acid.
4.Now that the plates are clean from the bad kind of electro chemicaly insoluble suphate (Crystalline), Time to now generate the good kind of sulphate on the plates. DISCHARGE BATTERY TO THE LOWEST IT CAN GET.
5.Drain out the epsom salt solution from the battery. Leaving it there. Interferes with the +ve plates and hinders the evolution of H+ ions necessary for electro chemical action
6. Put back your OEM eectrolyte( Dont worry if it looks dirty) -The thing is that we must restore battery chemistry to as close to OEM as possible.
7.Charge the battery with the original acid as eletrolyte.
You will get maximum 85 % lost cranking power back & add max 50% more life to the battery
Thanks for your suggestions!
This method works everytime!
Caution though:
1. Never put Baking soda / Tap water into the battery, It Eats away the Porous (+ve)PbO2 Plate
2. Never fail to Completly discharge the battery before switching over the Epsom salt solution with the old acid. -Battery Amperage will drastically fall.
3. If It still cant crank over a cold engine, add some acid when batt is fully charged( use a hydrometer to ensure each cell gets on the scale)
By “fully discharge the battery” do you mean drain it until a reasonable cut-off of 10.6-11.1v or even less than that? Do you risk cell reversal if you go too low?
Drain it to it’s effective “empty” voltage, so around 11.6V for a lead acid battery.
I used MichaelM’s way and it worked PERFECTLY!! The battery would not hold a charge when I jumped started it and could drive it for 15 minutes and then try and restart it and nothing.
My battery is fairly large sized and required 1500 ml of distilled water and 375 grams of
magnesium sulfate.
I microwaved 500 ml of distilled water in a glass pyrex measuring cup for 4 min(600 watts adjust according) and then added that hot water to a plastic cup containing 125 grams of
mag sulfate. Then dumped the concentrated solution into a 5 gallon bucket.
That was repeated 3x which gives 375 mag sulfate used and 1500 ml distilled water.
If you dont have a digital scale, 125 grams is real close to 1/2 level cup of mag sulfate + about 2 extra teaspoons.
You will need a funnel and a pair of safety glasses.
When charging the mag sulfate solution in the battery, it took about 6 hours at 6 amps charge.
When the OEM solution was added back in to charge it only required like an hour at 6 amps.
added
Thanks for sharing Mike, good to hear you’ve managed to get your battery restored!
That is only a maximum of 36AH of capacity, assuming 100% efficiency,which is not possible,at best case scenario you get about 60% to 70% efficiency, that is a poor 24AH capacity, I don’t think this will start a car, did it work?
To those wondering, Google amp hours vs amps
I forgot to add that everything was stirred until dissolved with a rubber spatula.
I don’t know if heating the distilled water in a metal pan and using a metal whisk would contaminate the solution so metal items were avoided.
Interesting reading. Thank you all. I’m going to try this on some old batteries. One question though. Can one heat the distilled water to disolve the Epsom salts in, In a metal pot or use a kettle? I believe the whole reason for using distilled water is that it contains no minerals or charge. Will metal contact affect the magnesium solution? Someone microwaved the water in a pyrex beaker for perhaps that reason, which makes sense to me.
I have a riding lawnmower size battery out of my motorcycle. It was purchased new 2 years ago, used only a month and then placed on trickle charger for a year while i restored the old bike.
now it is too weak to start the motorcycle, doesn’t take a full charge well.
So…. I have drained the acid and replaced it with epsom salt solution and as of this writing it is on a 12 volt 2 amp charger. my charger is 12v 2A/6A model.
Q: Should i be charging at higher rate of 6A?
Q: Do i drain epsom solution after 24 hours and replace with acid?
You should charge it at the lowest current possible, so 2A. Try leave the Epsom salt solution in for a week or so and then drain it and replace it with the regular acid solution.
Thank you.
I do have a low amperage trickle charger, if lower amps would be better.
Yes, a trickle charge would be better if you’ve got one.
Was the battery left in freezing temps? If so, it’s not worth the effort. The plates become brittle and crumble apart. At least with the batteries I’ve attempted to revive.
I am refinishing an aluminum boat and it has 3 group 27 deep cycle and 2 group 24 standard starting batteries installed. They were fully charged when it was dismantled for painting 3 years ago. I was expecting to replace all batteries but I was getting excited reading all of the posts about the recovery process. Thanks for adding the comment concerning recovering frozen batteries not being worth the effort. Do you know what the difference is between the deep cycle battery and a normal one? Can you check the resistance of the battery with a multi meter to see if it is a good good candidate to recover? Thanks for al the info.
Hi Dave,
Great to hear you’ve been looking into reconditioning your batteries.
Briefly, deep cycle batteries generally have fewer and thicker plates than normal vehicle batteries. They have a higher reserve capacity but produce a lower maximum current than a normal battery. Battery health is quite a complicated science and there are a number of attributes which affect it, internal resistance being just one of them. This being said, I don’t think that you’d get any really meaningful information for recovery out of measuring their internal resistance unless you had some historic test data for a number of the same brand and model batteries.
It’s not an expensive process, so I’d give it a try on one or two batteries and see what results you get. You may be able to save yourself a significant amount of money. And if you can’t, then you’ve just wasted a box of Epsom salts. I would only proceed with replacing the electrolyte solution if you got reasonably good results from the Epsom salt solution.
I do not know much about chemistry but what happens to the magnesium in the Epsom salts. I assume the sulfur in the Epsom salts helps replenish the acid but I wonder what effect the magnesium has in the solution. Would it be better to replace the solution with fresh electrolyte after it has done its job of cleaning the plates.
I have a golf cart with six batteries.do I need fresh epsom salt fluid got each battery
Yes, you’ll need fresh solution for each. I’d do them all at once and mix up a bigger batch of solution.
The directions in the original post say to take the battery out of the vehicle. Is that really necessary? and if so, why?
Thanks for a great post and for all the interesting and helpful comments.
No you don’t have to take it out, it’s just usually easier to work on on a work bench or table.
There is a way to do this method and still get the battery back to 89 to 99 % life I have one I did that lasted 13 years after doing this and using a desulfnator inline while charging it..also I dumped all the acid put it in an old washer fluid bottle I cleaned out let the sediment settle and pulled off clean acid from the top added it back to the battery added the Epsom solution and charged the battery at 10 amps for 1 hour then changed it to 2 amp for 7 hours drained the fluid and hosed the battery out makin sure it was clean water coming out when I was done added new acid back and charged it on auto charge with a top line charger ….the charger also makes the difference you can not use a new charger or one with auto charge when you run the first phase…I been doing this since I was a kid was taught how to do it by my grand dad who was a mack truck mechanic …
Hi Grey Wolf,
Thanks for sharing this with us. It’s great to hear that these restoration methods have been used for a few generations already!
Hi
thanks for such an informative website. I am stuck up at home in lockdown since 4 months. my scooter battery Amco 12V , VRLA type lead acid battery didn’t charge up. scooter was not driven due to lock down for long time and then left in rains, first I suspected wiring short but later on checking the charging wire by kicking the scooter found that battery is at fault. connected a dc -dc 5V to +15V adjustable voltage booster for charging battery but the charging current is just 20 mili Amp. and the voltage dont sustain even while checking it rapidly drops down. Googled a lot and came across your website, so decided to desulphate!
I committed a mistake I didnt drain the battery of the initial battery water or acid in it. the design of the VRLA battery is some what makes us get a false impression that the water level is between the min and max levels marked in the outside. I checked by a flashlight from the side walls and saw the light coming out from the other end when I shook the battery the liquid inside was well within the marked levels. when I opened the battery , I was surprised to see that in each of the 6 cells the water level is very low, very shallow and I have been trying to charge it with my voltage booster since 3 days. Since the water itself is very low I didnt drain it. I added around 15 to 20 gm of powdered epsom salt in a cup of boiling water about 100 ml and injected it with syringe in each of the 6 cells. as I kept on pouring there was rapid effervescence and bubling with gas formation. which gas ? it was horrible something like chlorine or sulphuric acid itself. seemed as if the acid is boiling!
then i realised it was a bad idea. but somehow i thought to finish the process it was becoming difficult to breathe, I wore wet face masks and avoided to inhale as much as possible. later left it in a open area on the roof of my house. the droplets of water epsom solution at the mouth of each cell started turning green
my question what gas was it possibly sulphuric acid itself? is the battery still safe and shall I charge it to see if it can hold charge now? or will it blast off or something ? shall I take it to a professional garage? I have a garage who works from the backdoor somehow during lockdown, he shown me his charging set up a huge transformer power supply at 16.5 Volt !.
will it be safe to charge this battery at 16.5 V or shall I try charging at lower value ?
shall I drain the battery solution now ? or use it for a few days and later drain it or leave it in this state?
pls help
Hi Navin,
I haven’t had much experience with VRLA batteries, hopefully someone else here has. I do know that its normal for their level to be very low, they’re intentionally made this way. Is the battery an AGM or Gel Type? I would imagine that either way this restoration method is going to be inhibited in these battery types because their design intentionally limits the movement of the electrolyte, which is going to prevent your Epsom salts from mixing into the solution properly. Also, 16.5V is too high to charge a lead-acid battery safely, they should be charged at around 14V.
I have tried to charge my BMW X5 battery as per the following method
a.I made a solution of baking soda ie 500 g baking sode to 5 l of wate
a. I repeated the wash out process with baking soda twice.
b.I then made a solution of 500 g of Epsom salt to 5 l of Distell water.
c. Charged the battery for 6 hours.
d. Put it back in the vehicle and tried to start……did not start
e. The battery was charged to 12.4v
?? What am I doing wrong ?
Sunny South Africa
Hi Indran,
You need to retain some of the electrolyte solution during the reconditioning process. The liquid in a battery is an electrolyte which is diluted with distilled water, removing it and replacing it with just distilled water will result in a dead battery. You need to remove the water and add a 35/65 acid solution and then try the process again.
hi michael you didnt read indran post correctly…he didnt say that he only throw distilled water in but a mixture of distill water and epson salt
Hi Michael, I have ordered the Epson Salt, I already have pre-mixed acid, I’m going to give your method a try, I have one very bad battery, suspect the sulphate has dropped into the bottom so planning on doing your suggestion, fully charging the battery then removing all the acid & flushing with battery water, empty & refill with the battery acid mix, battery is 130AH leisure battery.
I had someone attempt to recondition my lead/acid golf car batteries. They said that they had reconditioned many batteries in the past. The batteries were about 6 years old. Long story short: It didn’t work. The golf car moved at about 5 mph for a block and then died. I am now shopping for sealed glass mat batteries. I don’t want to fool with the open batteries anymore. Glass mat batteries are expected to last 8 years in a golf car with a lot of use. I don’t golf but I find the unit nice for one to two mile runs around town several times a week. Our town has golf cart lanes on every street because it is considered a retirement community.
Wheelchair batteries would be great for that.
This is a most intriguing conversation and it has me wondering.
In running an off grid power system, there is a bank of deep cycle lead acid batteries in a utility shed, that each require replacement every 5 years roughly. An expensive and labor intensive headache, that could be greatly reduced if there were a way to refurbish the batteries. Furthermore, if the acid and decrystallize solution can be stored onsite and pumped directly into the batteries…
Couldn’t an automated system be setup to perform this de-crystalization and replacment of acid?
I guess in theory this may be possible. The problem is that each battery has six cells, which you need to individually drain to a certain level and then refill. So you’d need quite a complex dosing system to split off into 6 individually controlled drains and dosers for each battery as well as a way to measure the electrolyte level in each cell.
Thanks for this very helpful information. I have a car sitting there for over a month and I had to jump start that car. After some driving, i thought the battery was charged but I couldn’t start the car. After second jump start I drove for an hour and still won’t start next time. I bought a 4amp auto charger and changed 2 days and never reach full charge. I bought a different 1 amp charger and charged more than 3 days, still unable to complete the charge. I opened the 6 cell screws and noticed all cells except one have small bubbles when charging. Does this mean this cell is dead? Can I still follow this procedure to repair this battery? if so, since all cells are individually screwed, can I just do these steps on the one cell that doesn’t make any bubbles?
Where can I get the small vent caps? Rubber very simple but I lost one.
I don’t think you can buy them separately. Your best bet is from another battery or scrap.
My solar battery reads 13V when I test it but it is not pushing any current. What could be the cause? Can I just add distilled water and recharge it?
If you put a load on the battery does the voltage drop significantly? It seems like your battery is dead, you should try reconditioning it as per the instructions here.
Hi ,we are working about repair and revive seald battery, especially UPS battery, my question is, can we change the plates of battery ,means when we cannot achieve good results from charging and chemical Procedure we open the door of battery and change defective plate ,thank you
I doubt you’d be able to source replacement plates and they’re generally sealed into the battery when it’s made up.
Yes, battery plates replacement is a common procedure in 3rd world countries like Pakistan. You can look for a battery repair shop near general bus stand of your city.
You buy a car and use its original battery for 2 years.
Replace with a 2nd battery and trickle recharge the 1st for the next 2 years.
Then swap them again and trickle recharge the 2nd battery for 2 years.
Then swap them again and trickle recharge the 1st battery for 2 years.
And so on and so forth. I used a 4 amp charger that automatically adjusted the charge rate down as the battery charged., used intermittently 3 months at a time. The longest use I obtained from a battery was a 1972 Dodge Dart Swinger with 225 cu in inline “slant” six. Its original equipment battery lasted for 6 years and the “alternated” replacement battery lasted for 8 years and was still in the car when resold. It continued for another 2 years according to the 2nd owner, Trickle maintenance charging works. Never added MgSO4 Epsom salt, never dumped acid or added acid. Never even checked the voltage. I just used distilled water without any additives (watch out, some have additives!) I kept the battery in the basement where the temp was between 50F to 65F year round. So, 14 years for two batteries (actually 16 years) with no failures to start in New England’s sometimes below 0 temperatures. And, I only bought one battery in 14 years. IMO, a 2 amp charger is all you ever need. Long slow charging done frequently out of the car works better than charging in the car, but charging in the car is better than not charging at all. It doesn’t help with lead sulphate unless you disconnect the negative pole. Microprocessors in modern cars leak current continually for anti-theft and some leak current through accessories unless you a certain to TURN THEM OFF before you shut off the car’s engine.
It seems to me that there are a lot of people out there that have a problem reading and following instructions.
Yes. It’s because of the “no child left behind” experiment brought to us by the “everyone gets a Trophy, even the Losers” section of society.
Thank you, George Bush!
Why not replace the MgSO4 solution with fresh electrolyte after the cleaning process? Any experience here doing this?
You can replace the solution with fresh electrolyte after cleaning, a couple of people in the comments have done so and reported good results.
I have always use baking soda and regular water to remove the crystals from the battery. Then I use epsom salt and distilled water as the battery acid solution. That solution remains in the battery and I don’t replace it with sulfuric acid. It seems to work fine.
Seems to me if you add the water with the Epsom salt as soon as it’s done boiling in all crystals have dissolved to the battery it charges faster and gains acidity faster just saying!
If your battery was discharged over a few days (still applies up to a few weeks) to 0V, here is some info that may help IF you have the capability to current limit your voltage supply, i.e. current limited voltage supply. Note: My car battery charger wouldn’t charge the battery because it couldn’t detect a voltage (it was 0 V) and I knew better to try and jump start it with another vehicle because it could damage the alternator.
Another note: If your battery charger doesn’t work because the battery is at 0 V and you don’t have a current limited supply but you do have a voltage source of 15 V or less you can prime your battery to a non 0 V state, now you can plug it in to your battery charger.
Pre-check: Pull your caps off of your battery (use proper PPE) and make sure all of the cells are fully covered with liquid. If not, add distilled water only being sure not to over fill.
1. Set your current limit to 1 A. You can use up to 1.8 A if you wish, batteries are just more forgiving when at lower currents, this is my preference only.
2. Set your voltage to 13.5 V.
3. Turn the supply on, your current should be limited based on your setting and you will slowly start to see the voltage increase. This may take 7-10 hours or more based on your current limit. See this link if interested: https://batteryuniversity.com/learn/article/charging_the_lead_acid_battery
4. When 13.5V is reached the current start to drop off until it reaches a lower steady state. Your battery is fully charged.
5. Reverse the soft sulfation. Set your voltage supply to 15V with a current limit of 0.2 A (200 mA).
6. Turn your supply on for 24 hours. See this link, paragraph 6, if interested: https://batteryuniversity.com/index.php/learn/article/sulfation_and_how_to_prevent_it
7. Turn off supply, re-install battery.
I’m not sure if I’m right about this but I do believe when you apply a current to a absent salt solution The Epsom salt is converted to sulphuric acid. Is in the process of that happening the Epsom salt takes electrons from the sulfides on the plates. That releases the salt fides from the plates and restores the battery. If I’m not positive if that’s how it works if I am wrong I would appreciate if somebody could correct me that would help me out on several levels.
I have done this with baking soda to clean out and used regular tap water . Filled the battery shook and dumped until no more black suet came out. Then let drain upside down. Added 16 oz of epsom salt to a 2 quart bottle of distilled water and dumped into battery. No need to boil cause as you charge it will boil anyways. Charge for 24 hours maybe 48 if needed. Hookup battery to car leave head lights on and let the battery drain till the lights start to dim. Then recharge the battery for another 24 hours. Then good as new. Seems like a lot of work but this way will yield best results.
HELP! I got a 12v marine deep cycle type 24 battery for free. Owner said it was dead. I drained all the fluid that was left in it (not much) and then filled the cells with a distilled water/ epson salt solution until I could just see some solution. (I put as much epson salt into the boiling distilled water until it wouldn’t disolve anymore). I left it like that for about 5 hrs or so. (no charging). I checked it with my multi-meter and it reads 34.2 volts! I thought my multi-meter is going bad so I checked 3 other deep cycle batteries I have that are topped off and they all read 13.1 volts. I then checked the type 24 again and again it reads 34.2 volts! How can this be?
Question?: do we need sulphuric acid(if so how much?) or simply drain the epsom salt and just pour in distilled water.thank you
Yes, you need either the sulphuric acid (can’t tell you how much) or the epsom salt mixture can be left in the battery instead. Water will not work. I use the cheap Leroy-Merlin swimming-pool acidifier 35% sulphuric acid.
Hello. I have an industrial forklift battery that is “dead”. I could use some guidance in rejevenating it. May you help?
Can i use magnesium sulphate 7-hydratear instead of Epson salt?